- Studies
- Overview DFG-Projects
- Legal matters
- Data Protection
- Quality Management
- CTIS Management
- IT and Information Security
- Fees
- Meeting dates
- About us
- Contact
Documents for Other Studies are to be submitted solely through our internet platform
Please note the following information regarding our online platform:
- Access data to our internet platform will be automatically erased six months after the last login. In this respect, please note our Data Protection Declaration.
- The online platform accepts PDF files only.
- The files may not contain any special characters except for the hyphen (-) and the underline (_). A period (.) is also considered to be a special character.
- The submitted documents may not contain any links to websites or cloud storage sites, from which further documents are to be downloaded. The complete documentation must always be submitted to the Ethics Committee for review. Our office employees will not follow links.
- NOTICE: At present, our online platform does not offer a dedicated option for the notification a new study. We are currently working on adding this functionality. In the meantime, please use the “Nachtr. Änderung” option to notify a new study to the Ethics Committee.
New application
Documents to be submitted (each as a separate PDF):
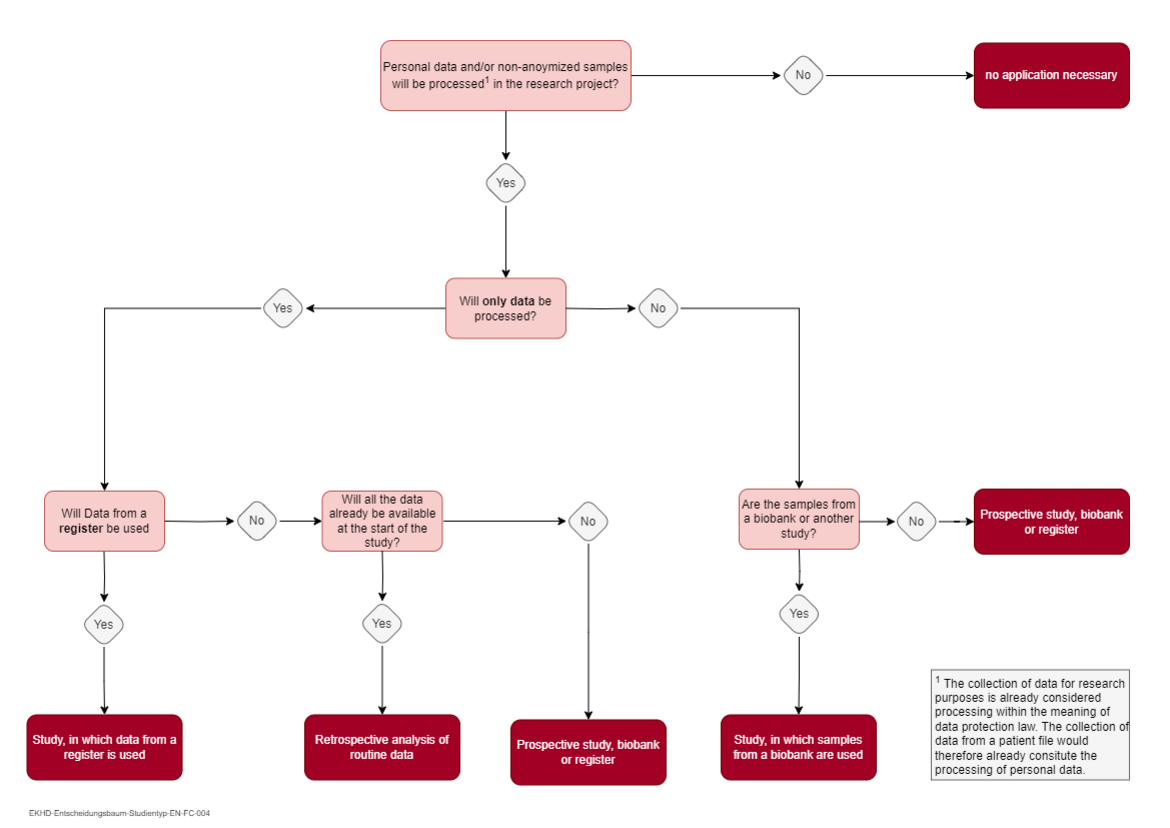
Please select the correct application type from the options below. If you are unsure which application type matches your research project, you can use the decision tree under the options for support. You can download a PDF version of the decision tree by clicking on the image.
Detailed information on the structure of a study protocol and on many special cases (e.g. studies without study-specific consent, studies with medical devices, studies with minors, etc.) can be found in the guide for other studies (please note: the guide for other study is only available in German).
On the pages dedicated to the four application types, you will find templates for study protocols as well as for an information sheet and consent form. You can use these templates to prepare your application . Alternatively, you may also use the eTIC tool recommended by the Association of Medical Ethics Committees.
Amendment
An amendment is a change that is undertaken after the commencement of the study (i.e., after receipt of the statement of the Ethics Committee). No distinction is made between substantial and non-substantial amendments for Other Studies. Therefore, formal amendments of the study documents should also be submitted to the Ethics Committee.
Amendments are to be distinguished from so-called content-related revised submissions or formal revised submissions (see below).
amendments to notified studies ("one study - one vote")
The "one study – one vote" principle also applies to amendments. Accordingly, the Heidelberg Ethics Committee only needs to be notified of amendments to studies for which another Ethics Committee is the responsible Ethics Committee (within Germany) in the following cases::
- Addition of a study site under the responsibility of the Heidelberg Ethics Committee
- Deregistration of a study site under the responsibility of the Heidelberg Ethics Committee
- End of a study
- Change of the local Principal Investigator (PI)
- Modifications to documents previously submitted to the Heidelberg Ethics Committee as part of the notification (→ structured synopsis, short protocol, list of study centres)
This procedure also applies to research projects that have already been reviewed by the Heidelberg Ethics Committee and for which the Committee has accepted an existing vote from another German Ethics Committee. Confirmation of this acceptance can be found in the letter titled Berufsrechtliche Beratung ("Votum").
For studies where another German Ethics Committee is the “primary voting” Ethics Committee, but whose vote has not been accepted by the Heidelberg Ethics Committee, the local PI is advised to contact the Committee’s head office to clarify the appropriate next steps.
Amendments to studies for which the Heidelberg Ethics Committee is the responsible Ethics Committee (within Germany):
The following applies to every amendment: the changes made must be marked with the aid of the automatic change tracker of the word processing program used (“track changes mode”) and the date and the version of the changed document must be updated.
It is inadequate to merely use a color to highlight passages that have been changed!
You will find instructions for the common word processing programs on the Internet:
Documents to be submitted (each as a separate PDF):
- Amendment form
- Amended study documents with tracked changes using the “track changes mode”, with updated version and the date, and if necessary, signed
- For studies for which the HD Ethics Committee has adopted an existing vote of another ethics committee, the corresponding statement regarding the amendment of the same ethics committee must be attached. The same prerequisites apply as for an initial application
Please note
For amendments to studies submitted prior to June 2025, specific requirements apply depending on the study design:
For monocentric, prospective studies:
- The structured synopsis must be prepared and submitted.
For multicentre, prospective studies:
- The structured synopsis must be prepared and submitted.
- A list of the study centres must be prepared and submitted.
- The declaration of suitability of the study centre must be completed and submitted for each German study centre.
These requirements only apply if the respective documents were not already included in the initial submission or in a previous amendment..
As collecting declarations of suitability from all German study centres can be time-consuming - particularly for multicentre studies with numerous sites - it is permissible to submit these declarations as part of a separate amendment at a later stage.
Change in the principal investigator
Any change of the principal investigator must be submitted to the Ethics Committee as an amendment. The following documents are to be submitted for this purpose:
- Amendment form
- Signature page for the curent version of the study protocol, signed by the new principal investigator
- Updated Declaration of suitability of the study centre and consent of the clinic/institute head
- For studies that were only notified to the Heidelberg Ethics Committee: acknowledgment by the responsible Ethics Committee (within Germany) of the change of the local PI
If the study protocol has been formally adapted as part of the change in principal investigator, a tracked changes version using the “track changes mode” is to be attached.
Subsequent report of another trial site
The participation of another trial site in the study must be reported to the Ethics Committee as an amendment. Please note that the correspondence on the study is directed at the current principal investigator of the initial trial site within the jurisdiction of the Ethics Commission. However, the votes apply to all physicians within our jurisdiction and thus also include the staff of the new trial site. The following documents are to be submitted to report an additional trial site:
- Amendment form
- If applicable: updated list of participating study centres
- Declaration of suitability of the study centre and consent of the clinic/institute head
- For studies that were only notified to the Heidelberg Ethics Committee: acknowledgment by the responsible Ethics Committee (within Germany) of the additional study site
Insofar as the study protocol has been formally adjusted as part of subsequent reporting of another trial site , a tracked changes version using the “track changes mode” is to be attached.
If a list of participating study centers does not yet exist, this must be compiled. The template provided by the Ethics Committee can be used for this purpose.
Reporting the end of a study
The end of a study is to be reported to the Ethics Committee. Moreover, a final report containing a summary of the results and the conclusions of the study should be submitted to the Ethics Committee within one year after the study ends. The final report should also be submitted if the study was prematurely terminated. As an aid, you will find a sample template for a final report below. The following documents must be submitted to report the termination of a study:
- Amendment form
- Final report (submitted within one year after the termination of the study)
Revised submissions
General information (formal/ content-related)
The Ethics Committee makes a distinction between formal revised submissions and content-related revised submissions.
All revised submissions required by the Ethics Committee prior to an assessment of the respective application (initial application or amendment) during a meeting, are formal revised submissions. They will be requested by office employees either via email or in a letter of receipt ("Eingangsbestätigung").
Revised submissions in response to additional requests from the Ethics Committee after the assessment of your application during a meeting, are content-related revised submissions. You will be notified of such requests in a letter entitled “Inhaltliche Nachforderung”.
Independent of the nature of the revised submission, the following must be complied with:
- The revised submission must be made via the Online Platform of the Ethics Committee (see above).
- Only those documents that have been requested should be submitted at this time. Please refrain from submitting additional documents “pro forma”. This increases the workload for the office and can therefore lead to delays in the processing of your application.
Content-related revised submission
For content-related revised submissions the Ethics Committee requests that the following also be considered:
- Only those changes may be made to the documents which have been requested by the Ethics Committee in their letter (subsequent request for content-related revised documents ). All changes exceeding this may be submitted as a subsequent amendment (see above) after the conclusion of the assessment procedure (i.e., after receipt of the “vote”).
- Changes in the documents must be tracked using the automatic track changes mode of a word processing program (see above).
- For documents that must contain a version and/or a date (see list of documents to be submitted for an initial application), the version and/or the date must be updated. The designation of the version is left to the applicant (e.g., Version 1.0 → Version 1.1 or Version 1.0 → Version 2.0).
- In addition to the version with the tracked changes, one version in which the changes have been adopted, should be submitted (clean version).
- If the study protocol is changed, the revised version must be signed by the principal investigator.
Further templates
- Studies
- Overview DFG-Projects
- Legal matters
- Data Protection
- Quality Management
- CTIS Management
- IT and Information Security
- Fees
- Meeting dates
- About us
- Contact
Power of attorney for correspondence
Please note that for reasons of confidentiality, we may only direct our correspondence to the principal investigator and the persons designated by the principal investigator for this purpose. Please submit a corresponding power of attorney, if necessary. This power of attorney may be granted in an additional letter (see “Other Attachments”) or in the cover letter for the initial application. It must be signed by the principal investigator and contain at the least the following information: study title, email address of the authorized person.
This power of attorney applies solely to the study correspondence. It is not possible to authorize other persons to sign documents, e.g., the study protocol or application forms.



![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/9/b/csm_Firefly_Abstract_organic_flow_resembling_blood_vessels_shaped_as_a_tool__composed_of_glowing_651800_26778ea790.png)