- Studies
- Overview DFG-Projects
- Legal matters
- Data Protection
- Quality Management
- CTIS Management
- IT and Information Security
- Fees
- Meeting dates
- About us
- Contact
New applications for research projects in which data from existing registries or databases are used
Explanation of the study type
A register is a structured and standardised collection of data. The type of data and its processing can vary depending on the objective of a registry. Examples include registries on certain rare diseases, registries on certain types of medical devices, etc. Many registries are designed to be partially or completely prospective, so that new data can continue to be recorded even after the registry has been set up. In most cases, registries are already planned in such a way that the data collected can be made available for future research projects.
The documents listed below only apply to research projects in which only data from one or more existing registries is used, or in which data has been collected as part of another research project and can be made available for use by other researchers. If further data or samples are to be collected as part of the research project in addition to data from an existing registry/other research project, this is a prospective study and you need to use the forms and templates for prospective studies, biobanks and registers.
If only data from one or more existing registries or from another research project is used, study-specific information and consent can often be waived. However, this waiver must be justified in the study protocol, e.g. with reference to the consent already given for the collection, transfer and use of the data as part of the registry. The exact source of the data must be specified (i.e. the name and, if applicable, the reference number of the registry/ the other study must be stated).
Documents to be submitted (each as a separate PDF):
The following documents are mandatory for every application:
- Completed application form
- Study protocol according to checklist variant C, with version and date in the header or footer of the document
- Signature page for the study protocol (signed by the principal investigator)
- Only for multicenter studies: List of participating study centers including the locally responsible physicians, with version and date in the header or footer of the document
- Declaration of suitability of the study center and consent of the clinic/institute head
- Details of funding and cost absorption declaration OR confirmation from the thesis supervisor
- Information leaflet and consent form for the registry from which the data is taken
- Statement from the ethics committee for the registry from which the data is taken (→ not necessary if the registry was reviewed by the ethics committe of the Medical Faculty of Heidelberg University)
The following documents must be submitted if applicable to the research project applied for:
- Structured synopsis in German, with version and date
- Information leaflet(s) with version and date in the header or footer of the document
- Consent form(s) with version and date in the header or footer of the document
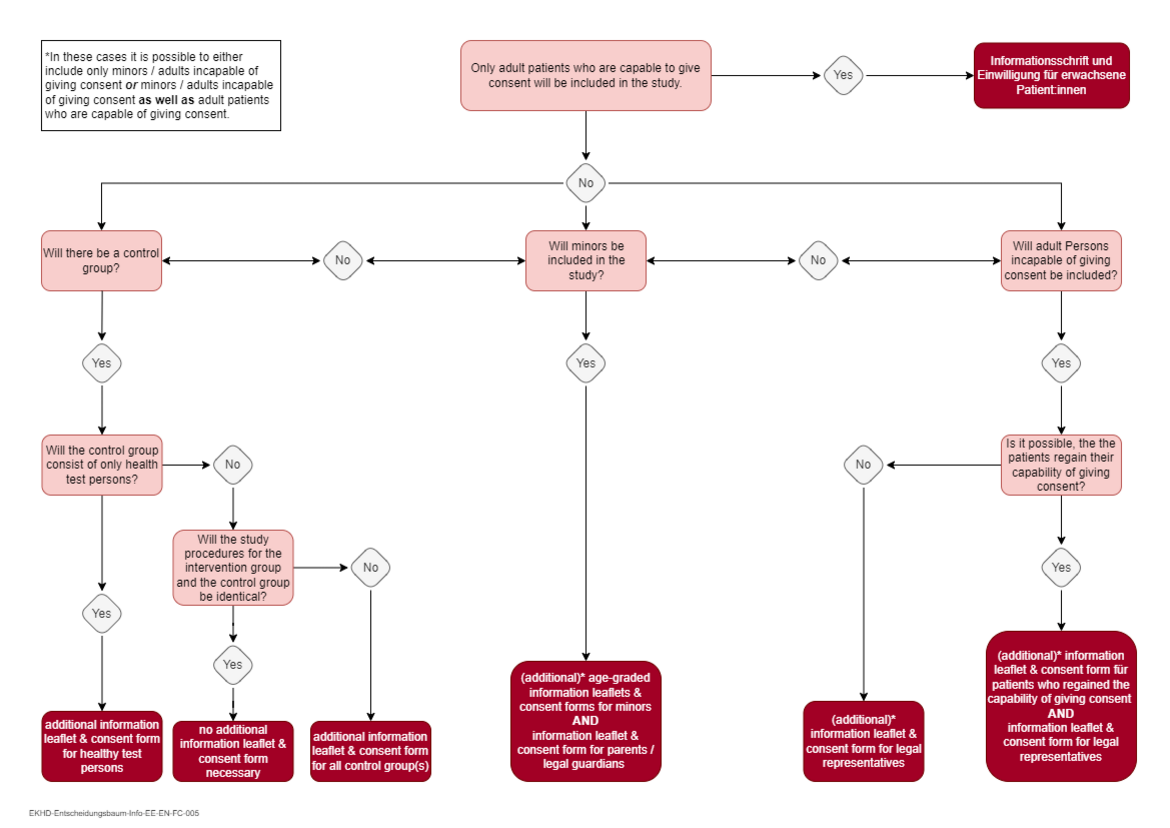
Please note: For studies with minors, age-graded information leaflets and consent forms for minors adapted to the respective target group as well as an information leaflet and consent form for parents/guardians must also be submitted. For studies involving adults incapable of giving informed consent, separate information leaflets and consent forms must be submitted for patients who regain the ability to give consent and for legal representatives. The decision tree at the end of the page can be used as support for the decision, which information and consent materials will be required. - Application for fee reduction/fee waiver
- Statements from other ethics committees
- Grant proposals (e.g. DFG, BMBF, etc.), with version and date in the header or footer of the document
- Cost calculation
- Contractual agreements with the study centre, including details of the compensation
- Power of attorney for study correspondence
Please note: A power of attorney is only required if persons other than the local principal investigator are to receive study correspondence. The authorisation must contain an email address of the additional recipients and be signed by the local PI.
The following table provides an overview of the documents that require a signature, a version and a date in the document.
| Document | Signature | Version | Date |
| Application form | Yes | No | No |
| Structured synopsis in German | No | Yes | Yes |
| Study protocol | Yes | Yes | Yes |
| Declaration of suitability of the study centre and approval of the clinic/institute head | Yes | No | No |
| List of participating study centres incl. locally responsible physicians | No | Yes | Yes |
| Cost absorption declaration / confirmation of supervisor | Yes | No | No |
| Information leaflet | No | Yes | Yes |
| Consent form | No | Yes | Yes |
| Grant proposal | No | Yes | Yes |
| Cost calculation | No | Can | Can |
| Contractual agreements with the study centre | No | No | No |
| Authorisation for study correspondence | Yes | No | Yes |
Decision tree for the required information leaflet(s) and consent form(s)
You can use this decision tree to determine which information leaflet(s) and consent form(s) you will need for your study. Please note that you may need several types of informed consent documents depending on the collective(s) that will be included in your study.
- Studies
- Overview DFG-Projects
- Legal matters
- Data Protection
- Quality Management
- CTIS Management
- IT and Information Security
- Fees
- Meeting dates
- About us
- Contact



![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/9/b/csm_Firefly_Abstract_organic_flow_resembling_blood_vessels_shaped_as_a_tool__composed_of_glowing_651800_26778ea790.png)