- Studies
- Overview DFG-Projects
- Legal matters
- Data Protection
- Quality Management
- CTIS Management
- IT and Information Security
- Fees
- Meeting dates
- About us
- Contact
New applications for retrospective analysis of routine data
Explanation of the study type
A study is retrospective if all the data to be analysed is already available at the start of the research project. If data collected after the start of the study is also to be analysed, the study is prospective and you need to use the forms and templates for prospective studies, biobanks and registers. To indicate that this is a retrospective research project, the study protocol should include information on the period in which the data to be analysed was collected (e.g. data collected in the period from 1 January 2020 to 31 December 2023). A study-specific participant information leaflet and consent form can often be waived for retrospective analyses of routine clinical data. However, this waiver must then be justified in the study protocol.
In the case of multi-centre retrospective research projects, it should be taken into account that the legal basis for a waiver of study-specific information and consent may differ depending on the federal state. This should be discussed in advance with all cooperating researchers.
Documents to be submitted (each as a separate PDF):
The following documents are mandatory for every application:
- Completed application form
- Study protocol according to checklist variant D, with version and date in the header or footer of the document
- Signature page for the study protocol (signed by the principal investigator)
- Only for multicenter studies: List of participating study centers including the locally responsible physicians, with version and date in the header or footer of the document
- Declaration of suitability of the study center and consent of the clinic/institute head
- Details of funding and cost absorption declaration OR confirmation from the thesis supervisor
The following documents must be submitted if applicable to the research project applied for:
- Structured synopsis in German, with version and date
- Participant information leaflet(s) with version and date in the header or footer of the document
- Consent form(s), with version and date in the header or footer of the document
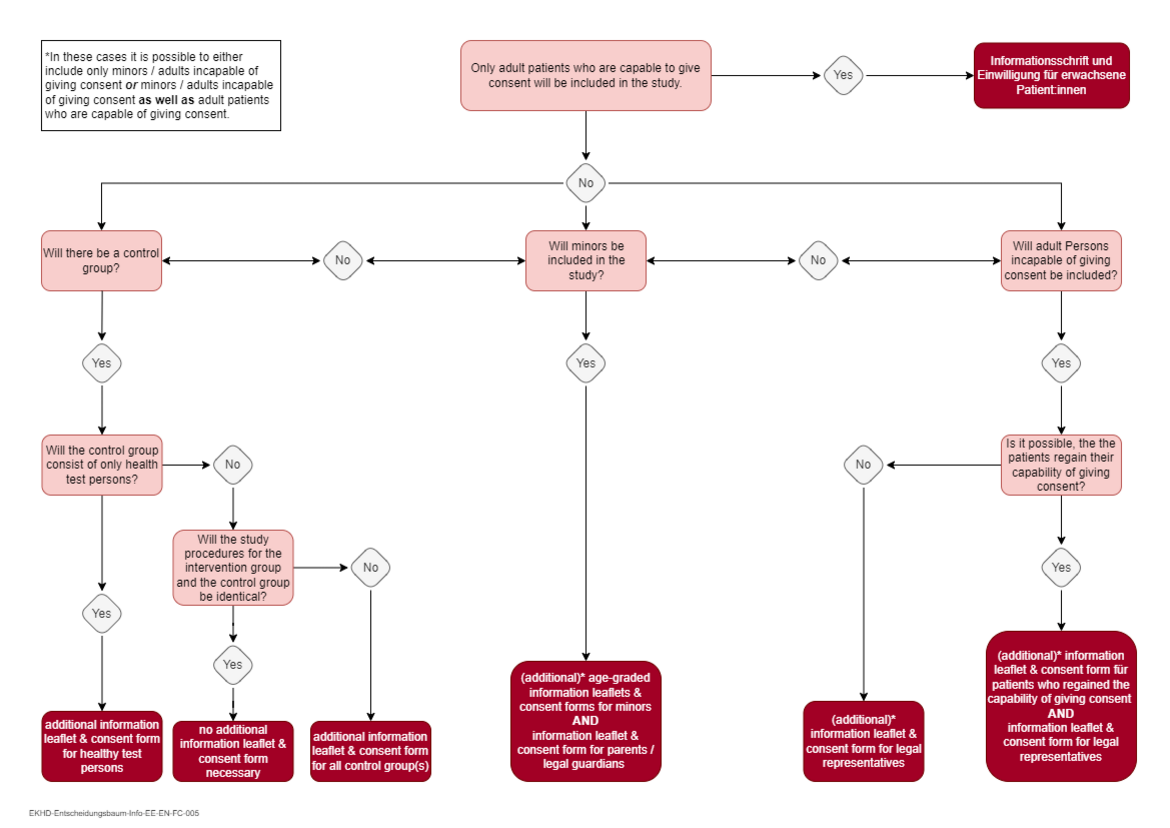
Please note: For studies with minors, age-graded information leaflets and consent forms for minors adapted to the respective target group as well as an information leaflet and consent form for parents/guardians must also be submitted. For studies involving adults incapable of giving consent, separate information leaflets and consent forms must be submitted for patients who regain the ability to give consent and for legal representatives. The decision tree at the end of the page can be used as support for the decision, which information and consent materials will be required. - Application for fee reduction/fee waiver
- Statements from other ethics committees
- Grant proposal (e.g. DFG, BMBF, etc.), with version and date in the header or footer of the document
- Cost calculation
- Contractual agreements with the study centre, including details of the compensation
- Authorisation for study correspondence
Please note: A power of attorney is only required if persons other than the local principal investigator are to receive study correspondence. The authorisation must contain an email address of the additional recipients and be signed by the local PI.
The following table provides an overview of the documents that require a signature, a version and a date in the document.
| document | Signature | Version | application |
| Application form | Yes | No | No |
| Structured synopsis in German | No | Yes | Yes |
| Study protocol | Yes | Yes | Yes |
| Declaration of suitability of the study centre and approval of the clinic/institute head | Yes | No | No |
| List of participating study centres incl. locally responsible physicians | No | Yes | Yes |
| Cost absorption declaration / confirmation of supervisor | Yes | No | No |
| Information leaflet | No | Yes | Yes |
| Consent form | No | Yes | Yes |
| Grant proposal | No | Yes | Yes |
| Cost calculation | No | Can | Can |
| Contractual agreements with the study centre | No | No | No |
| Authorisation for study correspondence | Yes | No | Yes |
Decision tree for the required information leaflet(s) and consent form(s)
You can use this decision tree to determine which information leaflet(s) and consent form(s) you will need for your study. Please note that you may need several types of informed consent documents depending on the collective(s) that will be included in your study.
- Studies
- Overview DFG-Projects
- Legal matters
- Data Protection
- Quality Management
- CTIS Management
- IT and Information Security
- Fees
- Meeting dates
- About us
- Contact



![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/9/b/csm_Firefly_Abstract_organic_flow_resembling_blood_vessels_shaped_as_a_tool__composed_of_glowing_651800_26778ea790.png)