Medizinische Zellbiologie - AG-Bas Orth
Research Summary
Metabolic plasticity and mitochondrial function in neurons
Synaptic activity drives calcium-mediated changes in neuronal gene transcription that are required for long-lasting adaptations of neuronal structure and function. These adaptations include – among others – synaptic plasticity, learning and memory, structural remodeling, and acquired neuroprotection. Our research focuses on a particular form of neuronal adaption, namely metabolic plasticity. We are interested in understanding how activity-dependent gene transcription controls neuronal energy metabolism and how this renders mitochondria more resistant to stressful conditions. To address these questions, we are using a combination of molecular biology, biochemistry and live imaging in primary hippocampal neurons, organotypic hippocampal slice
Current research
Transcription-dependent metabolic plasticity in neurons
We recently discovered a synaptic activity- and calcium-regulated gene program that drives changes in neuronal energy metabolism (Bas-Orth et al., 2017). In particular, we found that glucose metabolism shifts from oxidative phosphorylation towards aerobic glycolysis in synaptically activated neurons. This novel form of metabolic plasticity, which we termed ‘neuronal Warburg effect’, might be important for mitochondrial homeostasis and neuroprotection. We are now using metabolomics approaches to obtain a more comprehensive understanding of activity-dependent metabolic reprogramming beyond glucose metabolism. In addition, we are using live imaging of fluorescent nanosensors for glucose, l-lactate, ATP, and redox potential to study subcellular adaptations of neuronal energy metabolism.
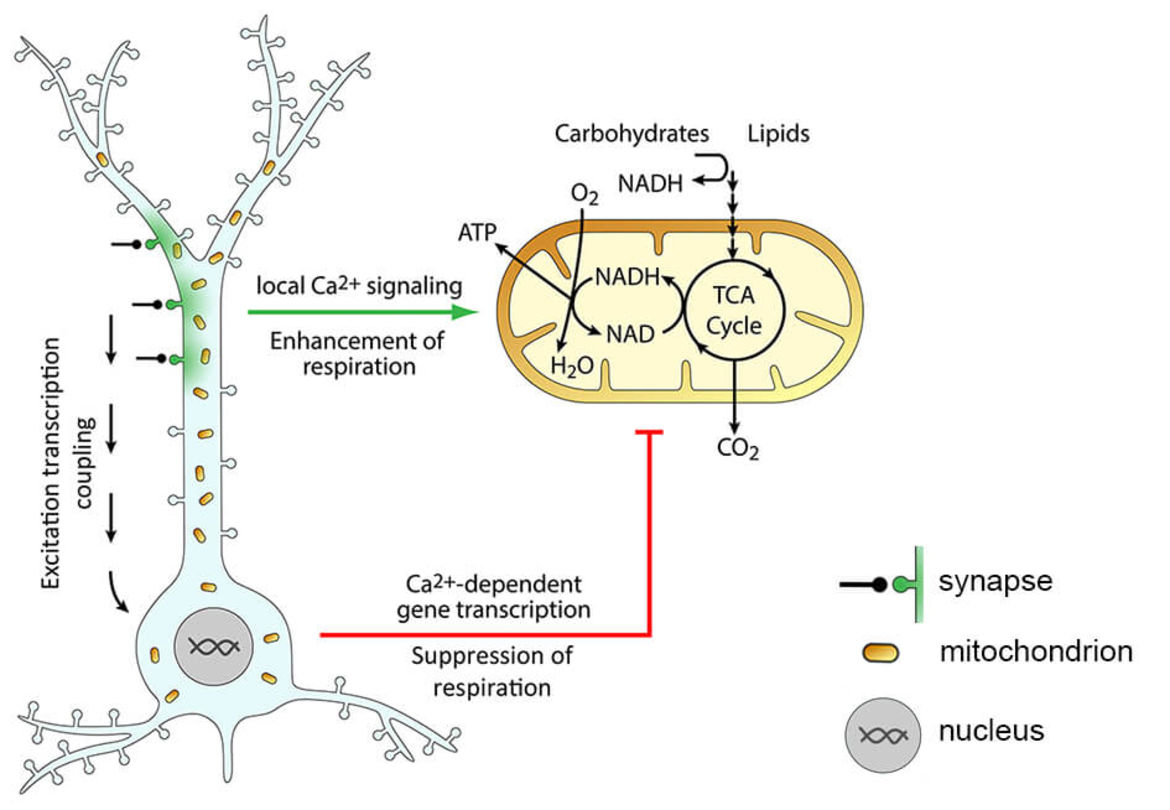
Figure 1. Activity-dependent metabolic plasticity in neurons. Synaptic activity leads to influx of Ca2+ (green) into the postsynaptic compartment, where it locally activates mitochondrial respiration to enhance ATP production. Strong or sustained synaptic activity triggers changes in gene transcription (excitation transcription coupling) that lead to a decrease of mitochondrial respiration, most likely to preserve mitochondrial homeostasis. Adapted from Bas-Orth et al., 2017.
Mitochondria as targets for neuroprotective strategies in Multiple Sclerosis
Mitochondria are integral for cellular function and survival. They generate ATP for cellular energy homeostasis, act as a Ca2+ sink and source to influence cellular Ca2+ signaling, and are key hubs for necrotic and apoptotic signaling networks. Accordingly, aberrant mitochondrial function is key to many neurodegenerative and neuroinflammatory diseases. In this project, we investigate the relationship between mitochondrial Ca2+ signaling, mitochondrial structure and function, and neuronal degeneration in the rat experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis and optic neuritis. We are using genetically-encoded fluorescent indicators to comprehensively analyze mitochondrial Ca2+ signaling and metabolic function in acute retinal slices from control and EAE animals. These novel indicators allow dynamic and quantitative imaging with single cell and subcellular resolution and thus provide information that cannot be obtained using conventional biochemical methods. Finally, by manipulating the expression levels of recently identified mitochondrial Ca2+ transporter proteins we aim at restoring mitochondrial Ca2+ homeostasis, metabolic function, and cell survival in the EAE model of multiple sclerosis.
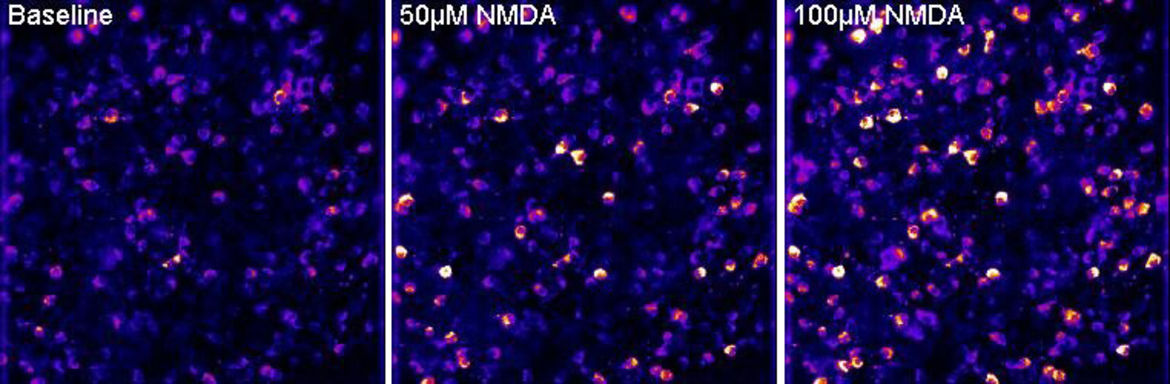
Figure 2. Live imaging of mitochondrial calcium signals in retinal ganglion cells. Single frames from a time-lapse recording showing mitochondrial calcium signals at baseline and after application of 50 µM and 100 µM of the glutamate receptor agonist NMDA to a live retinal flatmount. Recordings performed by Constanze Depp.
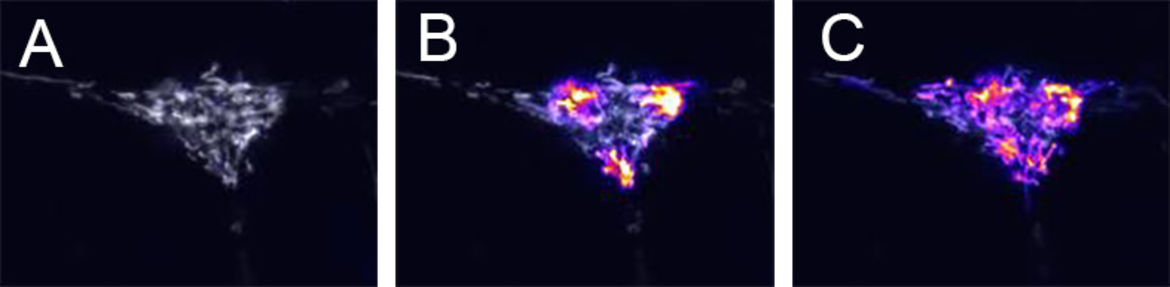
Figure 3. Live imaging of mitochondrial dynamics. A rat hippocampal neuron expressing mitoDendra2 is shown (A) before, (B) immediately after, and (C) 30 minutes after green-to-red photoconversion at 3 diffraction limited spots. Unconverted Dendra2 is displayed in grayscale, converted Dendra2 is displayed in firescale. Trafficking and fusion of mitochondria results in a redistribution of red Dendra2 protein across the cell. Recordings performed by Carlos Bas Orth.
Current funding
DFG FOR 2289 "Calcium & Neuroinflammation", TP7
DFG grant "Role of activity-dependent neuronal metabolic plasticity in brain aging"
Alumni
Shadi Albasset
Christian Bleischwitz
Dr. Angela Casaril
Sebastian Heesen
Jessica Hunger
Athanasios Katsalifis
Dr. Rolf Schmidt
Cristina Villegas Diaz