- Institut
-
Herz- und Kreislaufphysiologie
- Markus Hecker
- Thomas Korff
-
Hugo H. Marti
-
Forschung
- Zelluläre und molekulare Mechanismen der postnatalen Entwicklung des zerebralen Gefäßsystems
- Die Bedeutung der molekularen PHD-HIF Achse für den akuten Schutz und die langfristige Regeneration nach einem ischämischen Schlaganfall
- Charakterisierung und gezielte Aktivierung von NRF2-abhängigen antioxidativen Mechanismen beim akuten Schlaganfall
- Extrazelluläre Nukleinsäuren als Trigger neuroinflammatorischer Prozesse in akuten und chronisch degenerativen Erkrankungen des Zentralnervensystems
- Neuroprotektion und Neurogenese
- Blut-Hirn-Schranke
- Publikationen
- Personal
-
Forschung
- Andreas H. Wagner
- Neuro- und Sinnesphysiologie
- Lehre
- Zentrale Einrichtungen
- Bernard Katz Lecture
- Stellenangebote
- Aktuelles
Stress-mediated vascular remodeling
Our research group investigates responses of endothelial and vascular smooth muscle cells (ECs and VSMCs) to potentially harmful environmental conditions as may be caused by biomechanical, hypoxic or metabolic stress. Such stimuli evoke adaptive mechanisms to maintain cellular functions, which may, however, in the long run also contribute to detrimental remodeling processes of the vessel wall including its inflammation, stiffening, fibrosis and occlusion. In this context, we currently investigate 1) the regulation of EC and VSMC functions in the hypoxic lung and 2) mechanisms of VSMC foam cell formation in arteriosclerotic lesions.
Control of hypoxia-mediated stress responses of ECs and VSMCs by NFAT5 in pulmonary blood vessels and brain capillaries
We identified nuclear factor of activated T-cells (NFAT5) as major determinant for the transcriptional control of EC and VSMC responses to biomechanical, metabolic and hypoxic stress. In the hypoxic lung and brain (as it may occur during chronic obstructive pulmonary disease or stroke-mediated brain ischemia) NFAT5 is activated to modify the transcriptome for a condition-dependent adjustment of proliferation, migration and metabolism of ECs and VSMCs (an example is summarized below).
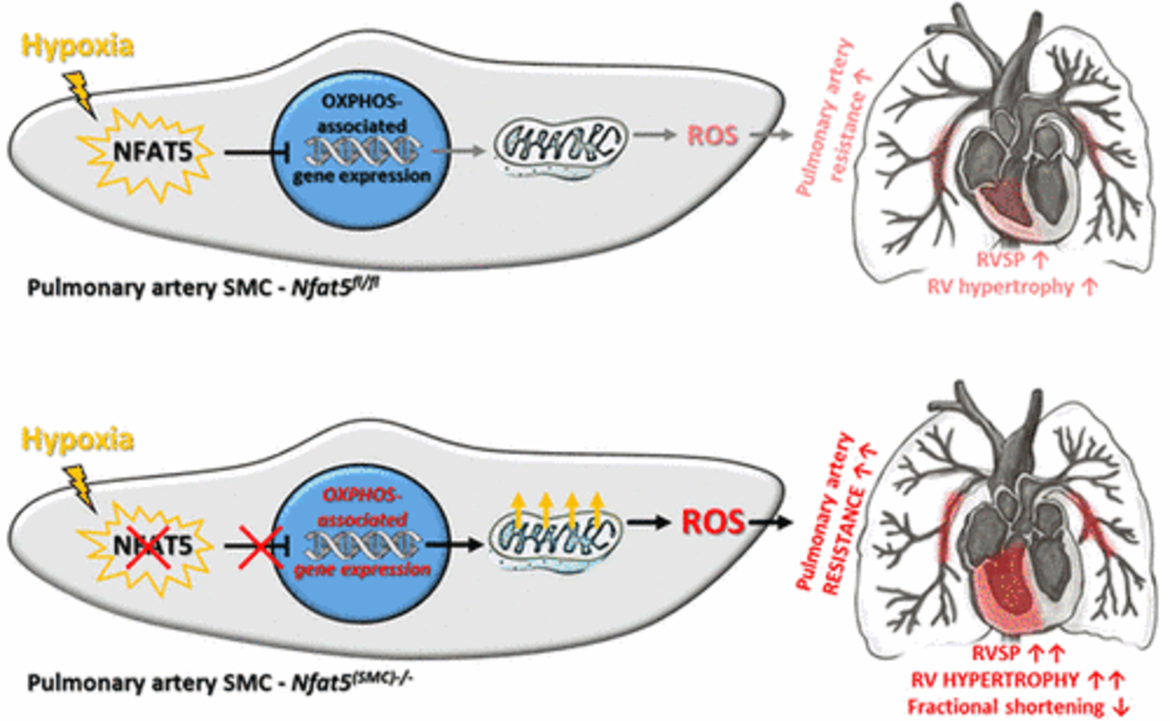
Impact of NFAT5 on VSMC function in hypoxic pulmonary arteries. NFAT5 is activated in hypoxia-exposed vascular SMCs and acts as a general transcriptional suppressor of metabolism- and OXPHOS-associated gene expression. This functional feature is required to limit mitochondrial respiration and ROS production in hypoxic pulmonary artery SMCs and attenuates the ROS-dependent pulmonary artery resistance during the early response to hypoxia. This transcriptional mechanism exerts important cardioprotective effects by preventing a detrimental rise in right ventricular (RV) afterload, exaggerated RV hypertrophy, and impaired RV functions.
In this context, we explore mechanisms of stressor-specific activation of NFAT5 as well as NFAT5-mediated changes of the transcriptome of ECs and VSMCs by applying mouse models of lung hypoxia and ischemic stroke in combination with modern transcriptomics.
Regulation of VSMC foam cell development
Arteriosclerosis is characterized by the accumulation of cholesterol in the arterial wall leading to lumen narrowing due to plaque growth and thereby increasing the risk for ischemia, myocardial infarction or stroke. Arteriosclerotic plaques consist to a large extent of lipid-laden foam cells, which derive from VSMCs to a significant part. We are interested in delineating mechanisms promoting and maintaining the initial phenotypic shift of VSMCs on the level of their transcriptome and proteasome. Recently, we identified NFAT5 as a potential determinant protecting cholesterol-exposed VSMCs from intracellular accumulation of lipids.
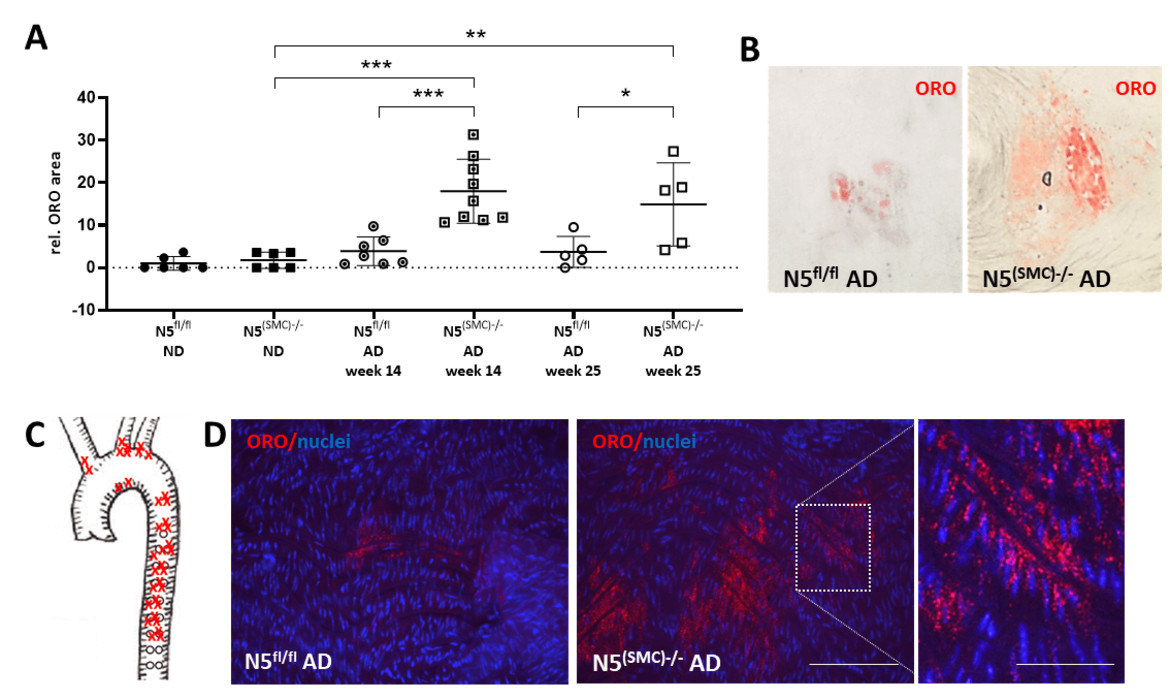
Analyses of blood cholesterol levels and lipid accumulation in the aorta of mice fed an atherogenic diet upon SMC-specific genetic ablation of Nfat5. Nfat5fl/fl (N5fl/fl) and Nfat5(SMC)-/- (N5(SMC)-/-) mice were fed an atherogenic diet (AD) for either 14 or 25 weeks or a normal chow diet (ND). Aortae were excised and stained with Oil Red O (ORO) to detect lipid-containing areas. The cumulative ORO-stained vessel area was recorded via light microscopy and quantified (A, ***p < 0.001, **p < 0.01, *p < 0.05 vs. control, n = 5-9, representative images are shown in B) and the location of ORO-positive areas in Nfat5(SMC)-/- mice was mapped (C). Confocal microscopy-based analyses of whole mount preparations of these aortae revealed ORO-positive droplets in the subintimal layers (D, scale bars: 100 and 40 µm).
- Institut
-
Herz- und Kreislaufphysiologie
- Markus Hecker
- Thomas Korff
-
Hugo H. Marti
-
Forschung
- Zelluläre und molekulare Mechanismen der postnatalen Entwicklung des zerebralen Gefäßsystems
- Die Bedeutung der molekularen PHD-HIF Achse für den akuten Schutz und die langfristige Regeneration nach einem ischämischen Schlaganfall
- Charakterisierung und gezielte Aktivierung von NRF2-abhängigen antioxidativen Mechanismen beim akuten Schlaganfall
- Extrazelluläre Nukleinsäuren als Trigger neuroinflammatorischer Prozesse in akuten und chronisch degenerativen Erkrankungen des Zentralnervensystems
- Neuroprotektion und Neurogenese
- Blut-Hirn-Schranke
- Publikationen
- Personal
-
Forschung
- Andreas H. Wagner
- Neuro- und Sinnesphysiologie
- Lehre
- Zentrale Einrichtungen
- Bernard Katz Lecture
- Stellenangebote
- Aktuelles




