- Institut
-
Herz- und Kreislaufphysiologie
- Markus Hecker
- Thomas Korff
-
Hugo H. Marti
-
Forschung
- Zelluläre und molekulare Mechanismen der postnatalen Entwicklung des zerebralen Gefäßsystems
- Die Bedeutung der molekularen PHD-HIF Achse für den akuten Schutz und die langfristige Regeneration nach einem ischämischen Schlaganfall
- Charakterisierung und gezielte Aktivierung von NRF2-abhängigen antioxidativen Mechanismen beim akuten Schlaganfall
- Extrazelluläre Nukleinsäuren als Trigger neuroinflammatorischer Prozesse in akuten und chronisch degenerativen Erkrankungen des Zentralnervensystems
- Neuroprotektion und Neurogenese
- Blut-Hirn-Schranke
- Publikationen
- Personal
-
Forschung
- Andreas H. Wagner
- Neuro- und Sinnesphysiologie
- Lehre
- Zentrale Einrichtungen
- Bernard Katz Lecture
- Stellenangebote
- Aktuelles
Forschung
Vascular Organoids for Aminal Welfare (3R)
Spheroid-based angiogenesis assays for the analysis of pro- and anti-angiogenic factors
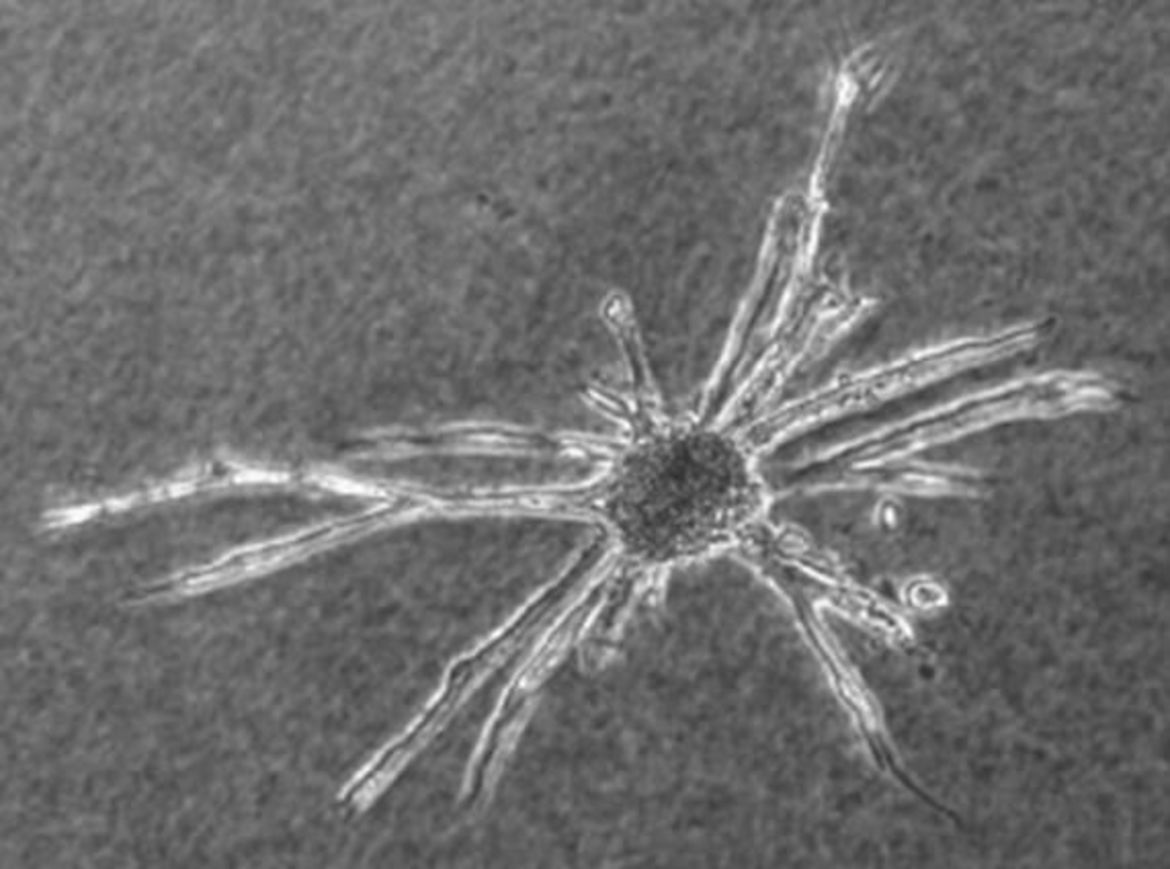
Spheroids are utilized as size- and cell number-defined focal delivery devices of quiescent ECs in various in vitro assays. For instance, embedding of the spheroids in 3D hydrogels and stimulation with growth factors results in angiogenic sprouting and network formation. Likewise, spheroids generated from VSMCs also support cellular quiescence and differentiation and are utilized for similar assays.The Figures below show examples of the formation of lumenized sprouts originating from collagen-embedded EC spheroids.

Outgrowing sprouts of neighbouring spheroids grow directionally towards each other to establish networks of anastomosing capillary-like structures (A, phase contrast microscopy). Cross sections of collagen gels embedded in paraffin show capillary-like, lumenized structures originating from EC spheroids (B-E). Depending on the plain of section, sprouting EC originating from lumenized capillary sprouts can be identified (D, arrow).
HUVEC spheroids embedded in collagen gels and stimulated with 50 ng/ml FGF-2 (E) or VEGF (F) form branching capillary-like sprouts originating from the spheroid body and invade the collagen matrix within 24-48 hours. The ‘tip’ cells extend filopodia into the collagen-gel (E, F, scale bars: 100 µm). Paraffin serial sections of sprouts, originating from collagen embedded spheroids, revealed that capillary-like sprouts form continuous lumen (G-L, scale bar: 20µm) dividing at the branching points (H, I). Time-lapse videos ( 20 h) showing the EC sprouting in detail are available for control-, VEGF (25 ng/ml)- and VEGF (50 ng/ml)-stimulated spheroids.
The number and length of the sprouts originating from one EC spheroid (determined as the cumulative sprout length [CSL]) indicate the level of a pro-angiogenic stimulation and are utilized as a basic parameter to quantify angiogenic activation of ECs. Consequently, spheroid-based EC sprouting can be exploited to screen compounds for their anti- or pro-angiogenic efficacy.

Collagen gel-embedded spheroids of different endothelial cell types were stimulated with 25 ng/ml VEGF for 24 hours (A, *p<0.05 vs. corresponding controls; CSL – cumulative sprout length). Representative spheroids of HMVEC are shown (B, C, scale bar: 200 µm). Collagen-embedded spheroids of HUVEC were stimulated with increasing doses of VEGF for 24 hours (D, *p<0.05 vs. control). Mean CSL was calculated for 10 randomly selected spheroids per experimental group. The inhibitors SU5402 (10 µM) and SU5614 (10 µM) were analyzed for their capacity to inhibit VEGF-induced (25 ng/ml, 24 h) HUVEC sprouting (E, *p<0.05 vs. control, #p<0.05 vs. VEGF). Representative spheroids are shown (F-H, scale bar: 100 µm). Mean CSL was calculated for 10 randomly selected spheroids per experimental group.
Analyzing EC-VSMC communication in vascular organoids
The spheroid-based technology is further exploited to form vascular organoids by mixing suspended ECs and VSMCs. During aggregation, they spontaneously organize within 24 to 48 h to form 3D vascular organoids composed of a surface EC monolayer that covers a core of VSMCs, thus representing an ‘inverted vessel wall’. Vascular organoids allow the investigation of EC-VSMC communication and can be formed by most types of human ECs and VSMCs, which develop a higher level of differentiation and quiescence as compared to (2D) standard cell culture conditions.

Aggregation and structural organization of vascular organoids generated from HUVECs and HUASMCs (A). Self-organization of suspended GFP-expressing ECs (A, top images, arrows: HUVECs) and VSMCs (A, top images, arrowheads: HUASMCs) were recorded by time lapse imaging. The endothelial cell marker CD31 was detected in organoids that formed after 48 h by immunofluorescence in combination with confocal imaging showing the cobblestone morphology of the EC monolayer on the surface (A, middle left image) and the core of CD31-negative VSMCs in the organoid center (A, middle right image). EC surface layer (bottom, left image).
CD31 staining of cross sections of vascular organoids (B) generated from combinations of aortic smooth muscle cells (HAoSMC), umbilical artery smooth muscle cells (HUASMC) and umbilical artery endothelial cells (HUAEC), umbilical vein endothelial cells (HUVEC), aortic endothelial cells (HAoEC), sphenous vein endothelial cells (HSaVEC), dermal microvascular endothelial cells (HDMEC).
- Institut
-
Herz- und Kreislaufphysiologie
- Markus Hecker
- Thomas Korff
-
Hugo H. Marti
-
Forschung
- Zelluläre und molekulare Mechanismen der postnatalen Entwicklung des zerebralen Gefäßsystems
- Die Bedeutung der molekularen PHD-HIF Achse für den akuten Schutz und die langfristige Regeneration nach einem ischämischen Schlaganfall
- Charakterisierung und gezielte Aktivierung von NRF2-abhängigen antioxidativen Mechanismen beim akuten Schlaganfall
- Extrazelluläre Nukleinsäuren als Trigger neuroinflammatorischer Prozesse in akuten und chronisch degenerativen Erkrankungen des Zentralnervensystems
- Neuroprotektion und Neurogenese
- Blut-Hirn-Schranke
- Publikationen
- Personal
-
Forschung
- Andreas H. Wagner
- Neuro- und Sinnesphysiologie
- Lehre
- Zentrale Einrichtungen
- Bernard Katz Lecture
- Stellenangebote
- Aktuelles