- Institut
-
Herz- und Kreislaufphysiologie
- Markus Hecker
- Thomas Korff
-
Hugo H. Marti
-
Forschung
- Zelluläre und molekulare Mechanismen der postnatalen Entwicklung des zerebralen Gefäßsystems
- Die Bedeutung der molekularen PHD-HIF Achse für den akuten Schutz und die langfristige Regeneration nach einem ischämischen Schlaganfall
- Charakterisierung und gezielte Aktivierung von NRF2-abhängigen antioxidativen Mechanismen beim akuten Schlaganfall
- Extrazelluläre Nukleinsäuren als Trigger neuroinflammatorischer Prozesse in akuten und chronisch degenerativen Erkrankungen des Zentralnervensystems
- Neuroprotektion und Neurogenese
- Blut-Hirn-Schranke
- Publikationen
- Personal
-
Forschung
- Andreas H. Wagner
- Neuro- und Sinnesphysiologie
- Lehre
- Zentrale Einrichtungen
- Bernard Katz Lecture
- Stellenangebote
- Aktuelles
Forschung
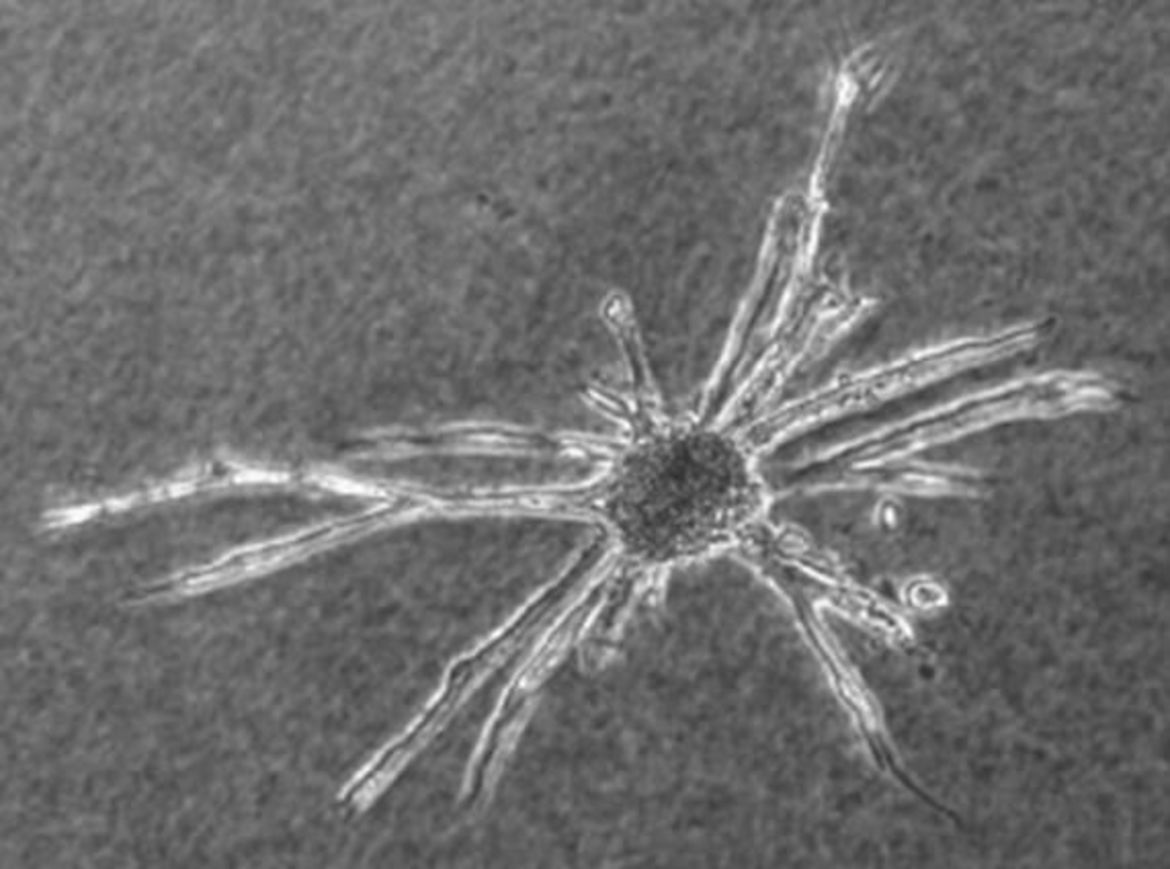
Vascular Organoids for Aminal Welfare (3R)
Due to the great interest in our spheroid based culture techniques we have put together frequently asked questions (FAQ´s) in a short catalogue. We hope that this catalogue can adress most of your technical questions and provide useful hints to bring the spheroids in your lab. If you feel that some other relevant questions should be adressed or if you have further problems to establish the spheroids in your lab do not hestitate to contact us.
Questions regarding the spheroid culture technique
- Which cell culture plastic do you use to generate spheroids?
Non adhesive cell culture plastic for suspension culture. We recommend 96-well-plates for suspension culture, U-form, cat#: 650185 from Greiner.
- Which cell types have been tested for their spheroid forming capacity?
So far, we tested HUVEC, HUAEC, HUSMC, HMVEC, human cytotrophoblast cells, BAEC, PAEC 10T1/2, C6-glioma and different epithelial cell types. All of the tested cell types performed well in spheroid based assays.
- How do you prepare the methocel stock solution which is needed for spheroid culture?
The preparation of methocel stock solution is very critical. If the concentration of methocel is too low or the solution is containing methylcellulose debris, single cells will stick to the wall and several small spheroids are formed in each well. We use methylcellulose from sigma (cat#: m-0512, 4000 centipoises). The methocel stock solution should have an extremly high viscosity. We autoclave the pure powder (6g) in a 500ml flask containing a magnetic stirrer (the methylcellulose powder is resistant to this procedure). The autoclaved methylcellulose is dissolved in preheated 250ml basal medium (60°C) for 20min (using the magnetic stirrer). Thereafter, 250ml basal medium (room temperature) is added to a final volume of 500ml and the whole solution is mixed for 1-2h (4°C). The final stock solution is aliqoted and cleared by centrifugation (5000g, 2h, room temperature). Only the clear highly viscous supernatant should be used for the spheroid assay (about 90-95% of the stock solution). For spheroid generation we use 20% of the stock solution and 80% culture medium.
- How do you generate the shperoids containig a precise number of cells?
The number of cells in a spheroid depends on the experimental procedure. If you want to analyze spheroids morphologically or biochemically (paraffin embedding, western blot, RNA analysis ect.), spheroids should contain at least 2250 cells. Spheroids which shall be embedded into collagen gels for functional anaysis (sprouting assays) should contain not more than 750 cells: For morphological/biochemical analysis trypsinize one petridish (55-75cm2) of a confluent EC monolayer. Suspend these cells in 10ml medium. Count the cells and seed a defined number of cells in methocel containing medium (20% methocel stock solution (see above), 80% culture medium [supplements/FCS: cell type dependent]). Distribute the medium to 96-well-plates (after trypsinization you count 3.000.000 cell in 10 ml, you need 4x96 spheroids (150µl/well) and one spheroid should contain ca. 2250 cells. Based on that, you need 2x1,5ml from the 10ml cell suspension. Dilute each 1,5ml in 30ml methocel containing medium and distribute it over 4x96 wells [1,5ml contains 450.000 cells; divided by 200 (exactly 2x96 wells) you get ca.2250 cells/well]). For morphological/biochemical analysis trypsinize one petridish (55-75cm2) of a confluent EC monolayer. Suspend these cells in 10ml medium. Count the cells and seed a defined number of cells in methocel containing medium (20% methocel stock solution (see above), 80% culture medium [supplements/FCS: cell type dependent]). Distribute the medium to 96-well-plates (after trypsinization you count 3.000.000 cell in 10 ml, you need 4x96 spheroids (150µl/well) and one spheroid should contain ca. 750 cells. Based on that, you need 2x0,5ml from the 10ml cell suspension. Dilute each 0,5ml in 30ml methocel containing medium and distribute it over 4x96 wells [1,5ml contain 150.000 cells; divided by 200 (exactly 2x96 wells) you get ca.750 cells/well]).
- How long can you culture the spheroids?
It depends on the cell type and culture conditions. HUVEC spheroids can be cultured for up to one week in media containing supplements like ECGS and FCS (10%). However, the cells in the center of the spheroids will undergo apoptosis. BAEC spheroids can be cultured for prolonged times (up to four weeks) in media containing FCS (10%). Because of their endogenous FGF-2 expression, the amount of apoptosis in the spheroid center is reduced.
- How do you harvest the spheroids?
We harvest the spheroids from 96 well plates using standard pipette tips (1ml, blue) transferring them into a 15/50ml tube. To enlarge the tiphole, 1-2mm (not more!) of the front of the tip is cut away.
- How fragile are the spheroids?
Whether the spheroids are fragile or not depends on the cell type you use and the culture conditions. For example, HUVEC spheroids are more fragile than BAEC spheroids. Generally, spheroids show a stable cellular organization. It is unlikely to disrupt spheroids by pipetting. However, cutting the pipette tip (1-2mm) before harvesting the spheroids should prevent their disruption. Note that EGTA-treatment induces disruption of HUVEC and BAEC spheroids.
Questions regarding the spheroid based sprouting assay
- How do you prepare the collagen stock solution needed for the angiogenesis assay?
To prepare a collagen stock solution you need 250ml steril filtered acetic acid diluted 1:1000 in aqua dest. (cell culture quality), 2 rat tails (stored at -20°C), 2x500ml 70% Ethanol. Place the rat tails for 20min in 70% ethanol, remove the skin. Wash the naked tails in ethanol. Break each second (depends on the length of the tails) vertebral and extract the tendons. Be sure to get clean tendons without attached connective tissue! When you finished that preparation, take all the tendons and place them in the second 500ml clean ethanol for 20min. Dry the tendons under the lamina air flow for 20-30min. Put the tendons in 250ml acetic acid (see above) and place them in the refrigerator for 48 hours shaking the solution at least two times each day. Aliquot the final solution in autoclaved tubes and centrifuge them at 4°C at 17.000g for 1h. Collect the clear supernatant. Be sure not to collect the debris!!! The clear supernatant/collagen stock solution can be stored in the refrigerator for at least 6 month. For equilibration, carefully mix 4ml of the stock solution with 0,5ml 10xHBSS and keep the mixed solution on ice for at least 15min. In most cases the collagen concentration of the stock solution is too high and the mixed solution will become a gel. In that case dilute the collagen stock solution (use 1:1000 sterile filtered acetic acid) and mix it with 10xHBSS as described above. Repeat diluting the stock solution till the mixed solution stays liquid. Now, the collagen stock solution is ready to use. To keep the EC baseline sprouting level low, it is advisable to prepare the stock solution at least 4 weeks before use (freshly prepared collagen by itself has the capability to induce sprouting of EC).
- How do you prepare the collagen gels for the spheroid based angiogenesis assay?
For 8 spheroid containing collagen gels (1ml/well in a 24-well-plate) put 4,5ml mixed collagen solution (0,5ml 10xHBSS + 4ml collagen stock solution) and 0,2N NaOH on ice. Harvest 400 spheroids in one 50ml tube and centrifuge (3min, 300-500g). Remove the supernatant and shortly scratch the tube over a rough underground to loosen the pellet (don´t let the pellet stay for more than 15-30min, otherwise the spheroids will stick together). Overlay (do NOT mix!) the pellet with 4-4,5ml FCS containing methocel stock solution (10-40%FCS, 90-60% methocel stock solution; FCS content depends on the cell type; note that the final FCS content in the gel is only the half of the concentration in this methocel solution). Right before use, neutralize the HBSS/collagen solution using ice cold NaOH (the phenol red pH indicator in HBSS should switch the color from yellow to flesh colored; mix fast and very carefully by inverting the tube to avoid polymerization. After neutralization keep the HBSS/collagen solution on ice. The neutralized collagen solution should be clear). Mix 4ml neutralized collagen with the 4ml spheroid containing methocel solution (again: do it fast and carefully). Divide the collagen/methocel solution on a PREWARMED 24-well-plate (8x1ml). Immediately place the plate into an incubator (37°C, 100% humidity). Let the collagen polymerize for at least 30min. Thereafter you can easily overlay the collagen gels with 100-200µl medium/supernatant. If you prepare the sprouting assay in the way described above, you must keep in mind that the supernatants to be tested first have to penetrate the collagen before they can affect the sprouting of the cells in the spheroids. To bring the spheroids in direct contact with agents to be tested use the following protocol: For each 1ml standard collagen gel, harvest 48 spheroids (half of a 96 well plate) and transfer them into a 15ml Falcon tube (48spheroids/tube). After centrifugation, remove the supernatant and shortly scratch the tube over a rough underground to loosen the pellet (don´t let the pellet stay for more than 15-30min, otherwise the spheroids will stick together). Overlay each pellet with 0,5ml methocel solution containing an appropriate amount of FCS (i.e. 20% FCS if the final concentration in the gel should be 10%; you also can use a medium containing only 40% methocel but the more methocel you use the slower the spheroids will sink to the bottom before the collagen becomes polymerized). Do NOT suspend the spheroids in the methocel solution! Add the agents to be tested to the methocel solution (again: do NOT mix!). Finally, each 0,5ml spheroids/agents-containing methocel solution is carefully mixed several times with 0,5ml neutralized collagen solution (see above) till the solution becomes homogenous and rapidly transferred into a PREWARMED 24-well-plate. Immediately after preparation of all collagen gels place the plate into an incubator (37°C, 100% humidity). Notes: For rapid collagen polymerization it is important that the plate is prewarmed to 37°C before the spheroid containin collagen/methocel-solution is filled in. Adequate mixing and rapid distribution of the final spheroid containing collagen/methocel-solution in a prewarmed 24-well plate is VERY important for homogenous dispersion of the spheroids in collagen gels.
- How can I decrease baseline sprouting in spheroid based angiogenesis assays?
To reduce the baseline sprouting, we culture the HUVECs as well as the HUVEC spheroids in medium containing all supplements (ECGS, FCS ect.). To keep the baseline sprouting low, the FCS content in collagen gels should be the same as in the culture medium. If your culture medium contains 10%FCS the collagen gel also should contain 10% FCS. You should never add any supplements in the collagen gel. This will dramatically increase the baseline sprouting. If the baseline sprouting is too high, just culture the spheroids for two days (instead of 24h) before use or decrease the FCS concentration (not below 5%). Note that some cell types endogenously produce FGF-2 (i.e. BAEC) and show a high baseline sprouting which can be decreased by adding FGF-2-neutralizing antibody.
- How long let you run the spheroid based sprouting assay?
Usually, up to seven days with BAEC or PAEC spheroids; up to three days with HUVEC spheroids.
Questions regarding paraffin embedding of spheroids
- How can spheroids be embedded into paraffin?
Spheroids are harvested (using standard pipette tips, where the tip was cut away [to enlarge the hole]) in 15ml tubes (at least 96 spheroids per experimental group) and centrifuged (3min, 200-500g). Subsequently, the supernatant is removed and the spheroid pellet is fixed for 12-24h (4°C) in 4% freshly prepared parafomaldehyde in HBSS or PBS. Centrifuge the spheroids again and remove the fixative. Wash the spheroids in water (10ml per tube) for 30min. Centrifuge again, remove the supernatant, suspend the spheroids in 70% ethanol and incubate for 45-60min (room temperature). Repeat this step with 85% ethanol, 96% ethanol and isopropanol. Remove isopropanol exept for 1,5ml. Carefully transfer the spheroid pellet together with 1ml isopropanol in a 1,5ml safe lock cup. Let the spheroids settle down and remove the supernatant. Fill the cup with 1,4ml 65°C temperatured low melting paraffin (melting temperature: 44-48°C), close the cup and place it into an oven (65°C) for 15min. Invert the cup several times to suspend the spheroids in the paraffin. Make sure that the tip of the cup is oriented towards the bottom and incubate it for 12h at 65°C. Place the tip of the cup into ice cold water till the paraffin becomes solid (the spheroids will be trapped in the solid phase) and discard the supernatant (note that only a small amount of low melting paraffin (ca 50µl) should remain in the cup. Otherwise it will disturb the solidification of high melting paraffin). Fill the cup with 1,4ml 65°C temperatured high melting paraffin (paraplast, melting temperature:56-58°C) and incubate it for 15min at 65°C. Invert the cup several times to suspend the spheroids. Because high melting paraffin will solidify rapidly during this procedure, make sure that all of the spheroids become suspended before the paraffin becomes solid. Orientate the tip of the cup towards the bottom and incubate it for 12h-24h at 65°C. Thereafter let the paraffin cool down to room temperature. Cut away the tip of the cup using a scalpel. If you carefully squeeze the cut tip, you easily can remove the paraffin plug inside with a tweezer. The spheroids are located at the front of the tip. Embed the paraffin plug frontside up in a paraffin block. Because the spheroids are located only in a thin layer inside the upper part of the paraffin block, control the paraffin sections using a microscope during sectioning to ensure that you don´t cut away the spheroids.
- Is it possible to embed collagen gels in paraffin to analyze sprouts originating from the spheroids?
It is possible but it will take several days (70%, 85%, 96% Ethanol each at least 12-24h, Isopropanol 12-24h, paraffin I (low melting) 24h, paraffin II (high melting) 24-48h). Another problem is (depending on the number of embedded spheroids) that you have to make a lot of sections to hit one or more spheroids/sprouts. Note that fixation of collagen gels in formaldehyd-containing fixatives makes the collagen more fragile and friable. For confocal imaging it is advisable to use whole mount protocols for immunhistochemistry and analysis or to exchange water in the matrix against glycerin. Collagenase will disrupt the entire structure of the matrix and most of the sprouts might be destroyed.
- Institut
-
Herz- und Kreislaufphysiologie
- Markus Hecker
- Thomas Korff
-
Hugo H. Marti
-
Forschung
- Zelluläre und molekulare Mechanismen der postnatalen Entwicklung des zerebralen Gefäßsystems
- Die Bedeutung der molekularen PHD-HIF Achse für den akuten Schutz und die langfristige Regeneration nach einem ischämischen Schlaganfall
- Charakterisierung und gezielte Aktivierung von NRF2-abhängigen antioxidativen Mechanismen beim akuten Schlaganfall
- Extrazelluläre Nukleinsäuren als Trigger neuroinflammatorischer Prozesse in akuten und chronisch degenerativen Erkrankungen des Zentralnervensystems
- Neuroprotektion und Neurogenese
- Blut-Hirn-Schranke
- Publikationen
- Personal
-
Forschung
- Andreas H. Wagner
- Neuro- und Sinnesphysiologie
- Lehre
- Zentrale Einrichtungen
- Bernard Katz Lecture
- Stellenangebote
- Aktuelles